Synthesis of Crystalline Metastable Oxides
Research in the Maggard lab aims to elucidate the underlying factors that can govern the kinetic stabilization of semiconductor 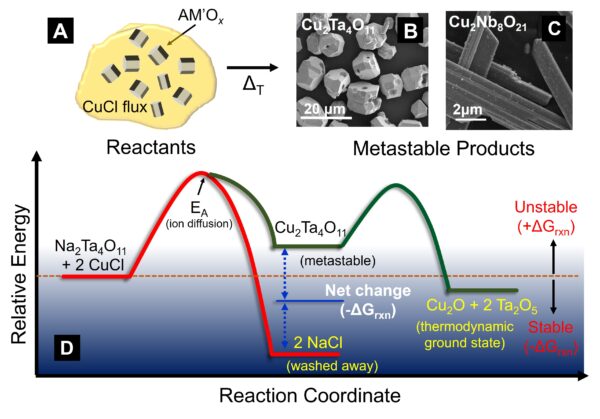 materials. Research thrusts specifically focus on metastable crystalline solids containing a combination of early (e.g., Ta, Hf) with either late transition-metal (e.g., Cu, Ag) or main group (e.g., Sn, Sb) cations. Crystalline solids within these chemical systems have been predicted to show desirable properties important for enabling their potential application as new lead-free ferroelectrics and as semiconductor materials for solar energy conversion. However, their synthetic preparation can be highly challenging and thus represents a key scientific bottleneck. Research is directed toward the discovery of new and productive synthetic approaches, such as using flux-mediated cation exchange reactions (shown in the figure). This type of low-temperature approach increases the range of metastable solids that can be experimentally attained by using reaction conditions which drive product formation while controlling ion diffusion and phase segregation, and thereby helping to inhibit decomposition. Structural characterization by X-ray and neutron scattering techniques is aimed at answering key questions regarding the formation and decomposition pathways of these materials.
materials. Research thrusts specifically focus on metastable crystalline solids containing a combination of early (e.g., Ta, Hf) with either late transition-metal (e.g., Cu, Ag) or main group (e.g., Sn, Sb) cations. Crystalline solids within these chemical systems have been predicted to show desirable properties important for enabling their potential application as new lead-free ferroelectrics and as semiconductor materials for solar energy conversion. However, their synthetic preparation can be highly challenging and thus represents a key scientific bottleneck. Research is directed toward the discovery of new and productive synthetic approaches, such as using flux-mediated cation exchange reactions (shown in the figure). This type of low-temperature approach increases the range of metastable solids that can be experimentally attained by using reaction conditions which drive product formation while controlling ion diffusion and phase segregation, and thereby helping to inhibit decomposition. Structural characterization by X-ray and neutron scattering techniques is aimed at answering key questions regarding the formation and decomposition pathways of these materials.
Recent Publications:
a) Gabilondo, E.A.; Newell, R.J.; Broughton, R.; Koldemir, A.; Pöttgen, R.; Jones, J.L.; Maggard, P.A. “Switching Lead for Tin in PbHfO3: Noncubic Structure of SnHfO3” Angew. Chem. Int. Ed. 2023, e202312130(1-9). * Selected as ‘hot paper’.
b) O’Donnell, S.; Gabilondo, E.; Koldemir, A.; Block, T.; Whangbo, M.-H.; Kremer, R.; Pöttgen, R.; Maggard, P.A. “Cation Exchange Route to a Eu(II)-Containing Tantalum Oxide”, J. Solid St. Chem. 2023, 124338.
c) O’Donnell, S.; Kremer, R.K.; Maggard, P.A. “Metastability and Photoelectrochemical Properties of Two Cu(I)-Based Oxides with Delafossite Structures” Chem. Mater. 2023, 35, 1404-1416.
d) O’Donnell, S.; Osborn, D.; Krishnan, G.; Block, T.; Koldemir, A.; Small, T.; Broughton, R.; Jones, J.L.; Pöttgen, R.; Andersson, G.; Metha, G.F.; Maggard, P.A. “Kinetic Stabilization of a Theoretically Predicted Sn(II)-Perovskite Oxide as a Nanoshell” Chem. Mater. 2022, 34(17), 8054-8064.
e) Gabilondo, A.; Newell, R.J.; Chestnut, J.; Weng, J.; Jones, J.L.; Maggard, P.A. “Circumventing Thermodynamics to Synthesize Highly Metastable Sn(II) Perovskites using Nano Eggshells” Nanoscale Adv. 2022, 4, 5320-5329.
f) Gabilondo, E.; O’Donnell, S.; Newell, R.; Broughton, R.; Mateus, M.; Jones, J.L.; Maggard, P.A. “Renaissance of Topotactic Ion-Exchange for Functional Solids with Close Packed Structures” Chem. Eur. J. 2022, 28, e202200479(1-6).
g) O’Donnell, S.; Smith, A.; Carbone, A.; Maggard, P.A. “Structure, Stability and Photocatalytic Activity of a Layered Perovskite Niobate after Flux-Mediated Sn(II) Exchange” Inorg. Chem. 2022, 61(9), 4062-4070.
h) Maggard, P.A. “Capturing Metastable Oxide Semiconductors for Applications in Solar Energy Conversion” Acc. Chem. Res. 2021, 54(16), 3160-3171.
Hybrid Semiconducting Photoelectrodes for Solar Energy Conversion to Liquid Fuels
The Maggard Lab is part of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), which is a Department of Energy Fuels from Sunlight Hub. CHASE’s mission is to develop molecule/material (hybrid) photoelectrodes for cooperative 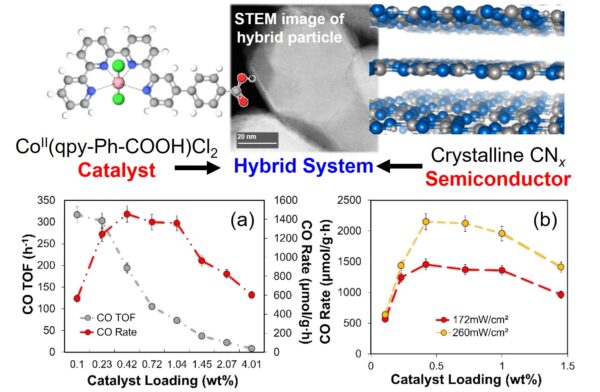 sunlight-driven generation of liquid fuels from feedstocks found in air: CO2 and H2O. The Center is pursuing these goals through three interconnected Thrust areas: Thrust I: Catalyst-Semiconductor Interfaces; Thrust C: Cascades Catalysis, and Thrust H: Catalytic Hybrid Photoelectrodes. Our research lab’s involvement in CHASE includes the investigation of molecular catalyst-photoelectrode material systems that function at high solar-to-chemical conversion efficiencies and stabilities in aqueous solutions for CO2 reduction, such as shown in the figure. Research efforts are aimed at unraveling the foundational relationships between the attachment of molecular catalysts on the surfaces of carbon-based materials (e.g., carbon nitride, graphene oxide, etc.) and their stabilites, turnover rates and selectivities for CO2 reduction when deposited onto semiconducting Si substrates. Interrogation of the molecular catalyst/carbon platform/semiconductor is conducted using a combination of spectroscopic (XPS, FTIR, TAS, etc.) and electron microscope techniques.
sunlight-driven generation of liquid fuels from feedstocks found in air: CO2 and H2O. The Center is pursuing these goals through three interconnected Thrust areas: Thrust I: Catalyst-Semiconductor Interfaces; Thrust C: Cascades Catalysis, and Thrust H: Catalytic Hybrid Photoelectrodes. Our research lab’s involvement in CHASE includes the investigation of molecular catalyst-photoelectrode material systems that function at high solar-to-chemical conversion efficiencies and stabilities in aqueous solutions for CO2 reduction, such as shown in the figure. Research efforts are aimed at unraveling the foundational relationships between the attachment of molecular catalysts on the surfaces of carbon-based materials (e.g., carbon nitride, graphene oxide, etc.) and their stabilites, turnover rates and selectivities for CO2 reduction when deposited onto semiconducting Si substrates. Interrogation of the molecular catalyst/carbon platform/semiconductor is conducted using a combination of spectroscopic (XPS, FTIR, TAS, etc.) and electron microscope techniques.
Recent Publications:
a) McGuigan, S.; Tereniak, S.J.; Donley, C.L.; Smith, A.; Jeon, S.; Zhao, F.; Sampaio, R.N.; Pauly, M.; Keller, L.; Collins, L.; Parsons, G.N.; Lian, T.; Stach, E.A.; Maggard, P.A. “Discovery of a Hybrid System for Photoctalytic CO2 Reduction via Attachment of a Cobalt Complex to a Crystalline Carbon Nitride”, ACS Appl. Energy Mater. 2023, 6, 10542-10553.
b) Genoux, A.T.-Y.; Pauly, M.; Rooney, C. L.; Choi, C.; McGuigan, S.; Fataftah, M.S.; Kayser, Y.; Suhr, S.C.B.; DeBeer, S.; Wang, H.; Maggard, P. A.; Holland, P. L. “Well-Defined Iron Sites in Crystalline Carbon Nitride”, J. Am. Chem. Soc. 2023, 145, 20739-20744.
c) Cypher, S.M.; Pauly, M.; Guadalupe-Castro, L.; Donley, C.L.; Maggard, P.A.; Goldberg, K.I. “Ethanol Upgrading to n-Butanol Using Transition Metal Incorporated Poly(Triazine Imide) Frameworks” ACS Appl. Mater. Interfac. 2023, 15, 36384-36393.
d) Pauly, M.; Kroger, J.; Duppel, V.; Murphey, C.; Cahoon, J.; Lotsch, B.V.; Maggard, P.A. “Unveiling the Complex Configurational Landscape of the Intralayer Cavities in a Crystalline Carbon Nitride” Chem. Sci. 2022, 13, 3187-3193.
e)Shang, B.; Zhao, F.; Choi, C.; Pauly, M.; Wu, Y.; Tao, Z.; Zhong, Y.; Harmon, N.; Maggard, P.A.; Lian, T.; Hazari, N.; Wang, H. “Monolayer Molecular Functionalization Enabled by Acid-Base Interaction for High Performance Photochemical CO2 Reduction” ACS Energy Lett. 2022, 7, 2265-2272.
f) Maggard, P.A. Ch. 2. Discovery and Development of Semiconductors for Photoelectrochemical Energy Conversion (Springer Handbook of Inorganic Photochemistry) Springer Nature, In Production, 2022.
